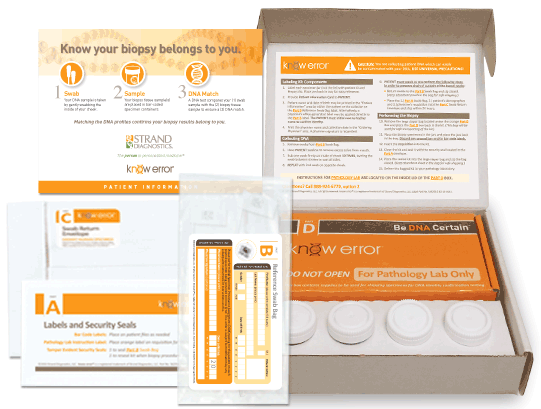
Professional Biopsy Specimen Kits
According to the American Cancer Society, about 1.7 million new cancer cases are expected to be diagnosed in 2015.1 In an effort to provide the most accurate diagnosis possible and facilitate proper treatment, hospitals and specialty practices around the country are embracing routine DNA Specimen Provenance Assay (DSPA) testing on breast and prostate biopsy tissues as a standard in cancer diagnostics.
We have designed a professional Biopsy Specimen Kit for physicians who conduct routine biopsies on other tissue types. Our laboratory staff has experience with a variety of other biopsy types including: bladder, cervical, colon and endometrial. Our professional biopsy specimen kits contain the components for confirming the assigned patient and ruling out contamination errors in the diagnostic process. Use your curser to pause (hover over) and advance the graphics (click the arrows).