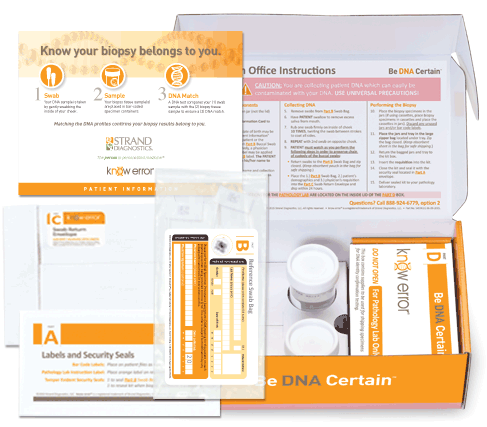
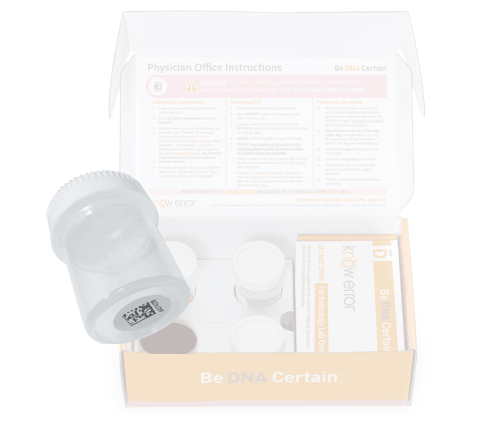
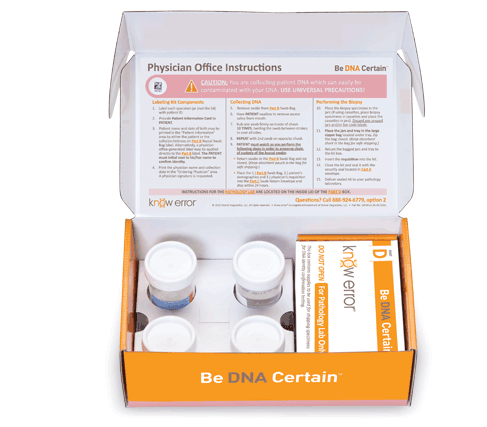
Professional Breast Biopsy Kits
According to the American Cancer Society, breast cancer is the second most common cancer among women in the United States, second only to cancers of the skin. In fact, it accounts for nearly one in three cancers diagnosed in U.S. women.1
Approximately 1.6 million breast biopsies are performed annually in the U.S. each year,2 about 20% of which will result in a cancer diagnosis.3 Given the complex nature of the biopsy evaluation process executed at this large of a scale, preventing diagnostic mistakes due to Specimen Provenance Complications (SPCs) is crucial to facilitate proper treatment and optimal patient outcomes. The patented know error® system utilizes DSPA testing, a buccal swab-to-biopsy tissue comparison, to rule out the possibility of specimen contamination errors and confirm the cancerous tissue(s) belongs exclusively to the patient being evaluated.
Professional kits contain components to confirm the assigned patient and ruling out contamination errors in the diagnostic process. Use your curser to pause (hover over) and advance the graphics (click the arrows).
The know error® system for breast biopsies first became available in early 2010 and is now utilized by industry-leading breast centers and radiology practices around the country. Testing is generally performed for any of the following diagnoses:
• All Malignant cases
• Atypical Ductal Hyperplasia
• Ductal Carcinoma In-Situ
• Invasive Ductal Carcinoma
• Invasive Lobular Carcinoma
• Atypical Lobular Hyperplasia
• Lobular Carcinoma In-Situ
• Intraductal Papilloma
• Paget’s Disease
• Phyllodes Tumor