Professional Prostate Biopsy Kits
Excluding cancers of the skin, prostate cancer is the most common cancer in American men–and also one of the most deadly. It is currently the second leading cause of cancer deaths, second only to lung cancer.1
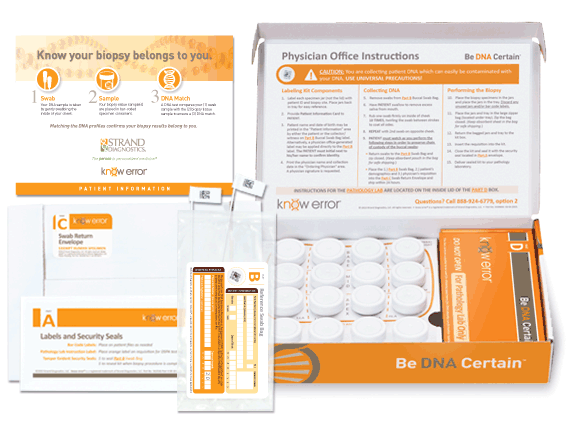
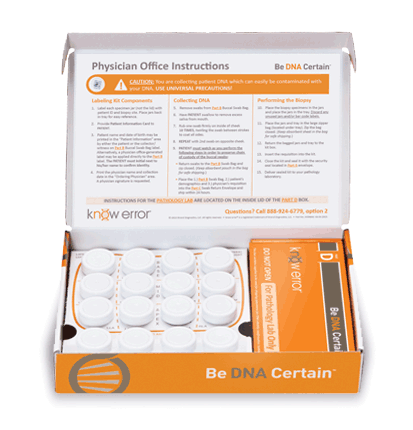
Approximately 650,000 prostate biopsies are performed annually in the U.S. each year2, about 30% of which will result in a cancer diagnosis. Given the complex nature of the biopsy evaluation process executed at this large of a scale, preventing diagnostic mistakes due to Specimen Provenance Complications (SPCs) is crucial to facilitate proper treatment and optimal patient outcomes. The patented know error®system utilizes DSPA testing, a buccal swab-to-biopsy tissue comparison, to rule out the possibility of specimen contamination errors and confirm the cancerous tissue(s) belongs exclusively to the patient being evaluated.
Professional kits contain the components for confirming the assigned patient and ruling out contamination errors in the diagnostic process. Use your curser to pause (hover over) and advance the graphics (click the arrows).
The know error® system for prostate biopsies was launched in the spring of 2009 and has gained widespread acceptance among leading urology practices throughout the United States. Hundreds of physicians have implemented this system as part of their standard of care to enhance diagnostic accuracy and safety for prostate biopsy patients.
1. American Cancer Society. Prostate Cancer Overview. http://www.cancer.org/Cancer/ProstateCancer/OverviewGuide/prostate-cancer-overview-key-statistics. Accessed January 10, 2012.
2. The Associated Press. GSK drug reduces prostate cancer risk, FDA panel says. WRALtechwire. http://wraltechwire.com/business/tech_wire/news/blogpost/8695765/. Posted November 29, 2010. Accessed January 9, 2012.
3. Pfeifer JD, Liu J. Rate of occult specimen provenance complications in routine clinical practice. American Journal of Clinical Pathology. 2013;139(1):93-100.