DSPA Testing for the Pathology Lab
Be DNA Certain™ that lab specimens are free from contamination and exclusively belong to the patient being evaluated.
Specimen Provenance Complications (SPCs) can occur outside a controlled laboratory environment even when automation technologies are employed through the Analytic Phase of testing and meticulous operating procedures are followed.
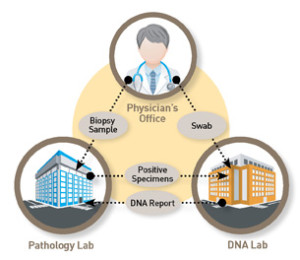
We has established the highest standard for specimen provenance testing with DNA Specimen Provenance Assay (DSPA), DNA testing. The DSPA test complements the laboratory workflow while protecting the pathology lab across the entire diagnostic testing cycle. DSPA compares a DNA profile of a patient’s reference swab to the DNA profile of the biopsy tissue(s) used in the pathology evaluation process to confirm a DNA match. A DNA match removes the doubt of specimen contamination and patient transposition errors confirming the specimen purity (i.e. free from admixture) which can otherwise confound test results.
How to order DSPA Testing
Please call 1-888-9Biopsy (924-6779), option 2. We are your trusted partner for fast, reliable results.
DSPA: Retrospectively (After the biopsy)
DNA Specimen Provenance Assay (DSPA) testing can be ordered after the biopsy process to eliminate the doubt of specimen contamination and patient transposition errors before adverse patient outcomes.
 Laboratory and medical professionals should order DSPA testing whenever one suspects a specimen provenance problem might exist such as:
Laboratory and medical professionals should order DSPA testing whenever one suspects a specimen provenance problem might exist such as:
• Areas of interest on slides or blocks
• Multiple medical samples found within the same specimen jars
• Fragments, floaters in histologic specimens
• Non-labeled specimen jars or slides
• Indiscriminate medical samples
• Resected tissue is discordant with the biopsy sample
To solve for these issues a patient DNA reference sample and tissue scrolls from the specimen(s) in questions are sent to us for DSPA testing to confirm (a DNA match) the samples are free from contamination (i.e. the sample is pure) and are ascribed to the correct patient.
DSPA: Prospectively (Routine for all biopsy patients)
When utilizing the patented know error® system on every biopsy patient as part of routine clinical care, unsuspected errors can be resolved before patient misdiagnosis. Know Error biopsy specimen collection kits (typically used at the clinic or treating physician’s office) encompass unique patient bar coding, forensics principles for specimen handling and DSPA testing to detect specimen provenance complications among the cancerous (malignant) tissue(s). Errors exist even when no error is suspected (occult errors).